Algae-Based Microrobots Clean Up Plastic Waste
Imagine you are but a piece of plastic, adrift in an endless ocean — a mere remnant of a once larger structure, the origin of which you do not recall. You are the product of many, many years of natural erosion, and yet you, invisible to the human eye, persist, the journal Advanced Functional Materials reported.
Moved by the ocean’s whims, your fate is likely to be consumed by whichever organism stumbles upon you first. But suddenly, a shadow looms overhead, and much to your disbelief, it is no fish or squid, but a green sphere covered in bits of black iron, moving towards you at unnatural speeds. You feel yourself being pulled towards it, and as you approach, you realize it’s also covered in many others like you.
Albeit a dramatic representation for effect, it hints at a remarkable development made possible by a team of researchers at the Central European Institute of Technology (CEITEC) at Brno University of Technology.
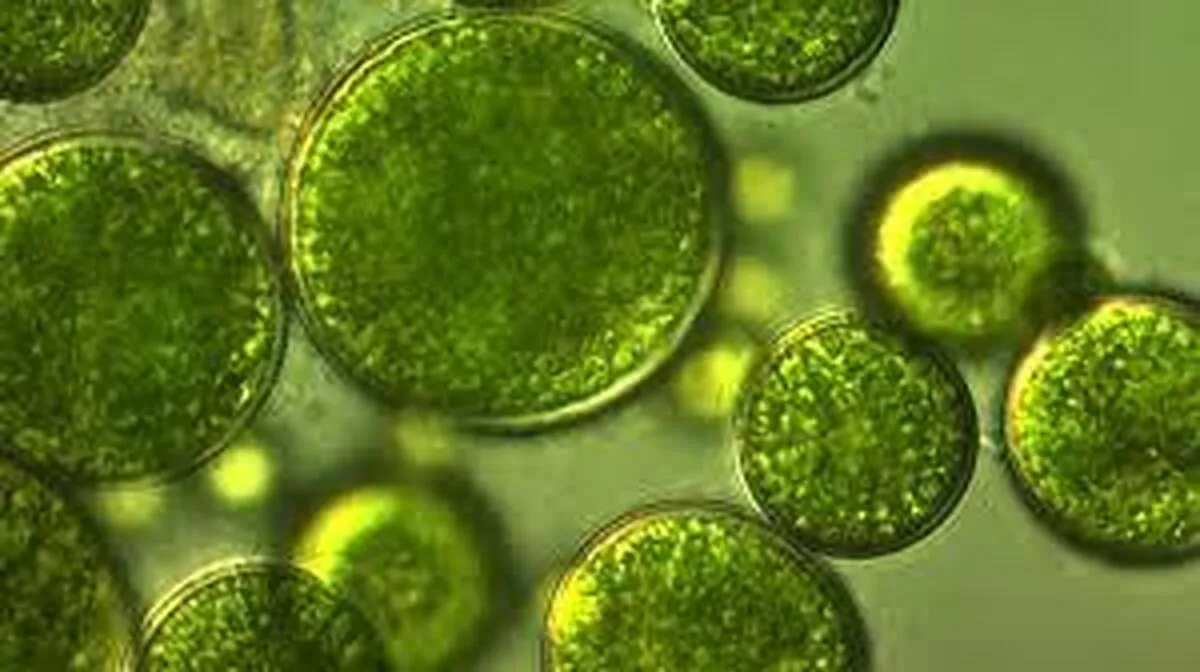
By decorating green algae cells with ever so tiny particles of black iron oxide — also known as magnetite — the team created magnetic algae robots that can be controlled from a distance to sift the most elusive of plastics from the waters.
But how does this system work, exactly? And why algae? To answer that, we need some context.
Let’s face it: we love plastics. They are cheap, flexible, and moldable, while also being very durable and light. This makes them extremely convenient materials for endless applications, and thus, their current widespread use.
However, these very advantages are also highly detrimental from an ecological perspective. Their rising levels of production, combined with their characteristic resistance to natural degradation, have led to huge quantities of plastic waste that will stay in the environment for anywhere between a few decades to several centuries.
Throughout this entire time, plastics in aquatic environments will break down into minute fragments that are categorized into microplastics (smaller than 5 mm) and nanoplastics (smaller than 1000 nm). These fragments are then ingested by fish and other aquatic organisms, causing physical harm, problems in digestion and reproduction, and potentially death.
They can also collect other pollutants present in the water, such as heavy metals and organic contaminants. These plastics and the contaminants they carry are transferred up the food chain, and scientists are still beginning to study what effects, if any, this might have on humans in the long run.
To try and clean this mess and mitigate the persistence of micro- and nanoplastics in the environment, researchers have been developing all sorts of solutions. Unfortunately, these generally suffer from being too complex or expensive to carry out, or simply weren’t efficient enough.
This is where the robots come in.
To better target these tiny plastics, a team of researchers at CEITEC came up with the idea of creating equally tiny janitors that they could control. These tiny robots are not like the conventional mechanical machines we’re used to but are themselves micro/nano-sized particles made up of a combination of various functional materials.
“I was thinking I could find one cheap and mass-producible material to replace expensive metals,” said Xia Peng, a researcher and Ph.D. student at CEITEC, and primary author of the current study published in the Advanced Functional Materials journal. “Then algae cells just came to my mind.”
Dubbing them “magnetic algae robots” or MARs, Xia and her team decorated cells of Chlorella vulgaris (a species of green microalgae) with eco-friendly magnetite nanoparticles, which enable the MARs to be manipulated using an external magnetic field. These algae are not only biodegradable, but they are also easy and cheap to mass produce.
Another advantage is that their surface is riddled with chemical groups called carboxylic acids, which carry a negative electrostatic charge. “The surface charge of MARs is negative due to the presence of [carboxylic acid] groups, while the surface charge of the micro/nanoplastics selected is positive, which promotes the electrostatic attraction of targeted micro/nanoplastics, allowing for their capture and removal,” explained Xia.
The negatively charged algae attract positively charged micro/nanoplastics and keep them “glued” to themselves. This is also how the magnetite nanoparticles, which are positively charged, can be attached to the surface of the algae cells for remote magnetic control without requiring any complex processing.
For their tests, the team used a positively charged fluorescent variant of the ubiquitous plastic polystyrene, whose size varied from 2 μm to 50 nm. This fluorescent form glows under specific experimental conditions and enables the team to measure the quantity of plastic removed from water samples by the MARs, including deionized, tap, rain, and lake water.
They added MARs to these contaminated water samples, sent them on predefined trajectories under magnetic guidance — picking up the polystyrene in their path — and then examined the treated samples by comparing their levels of fluorescence intensity before and after the treatment.
“The most significant findings,” declared Xia, “were the successful capture of micro/nanoplastics […] with high removal efficiency for both nanoplastics (92%) and microplastics (70%).”
Not only that, but MARs could be recycled for further use by washing off the captured plastics. Small amounts of their magnetite coating were also washed away, but they still preserved around 80% efficiency for capturing nanoplastics and 54% for microplastics even after five cycles of washing, after which they could simply don a fresh coat of magnetite and be back to full potency.
“MARs could potentially be tested in salt water since their magnetically driven movement is not affected by salinity,” mentioned Xia. “However, the study is still in the initial stage.
“It’s important to further study the biodegradability and potential long-term environmental effects of these nanoparticles to ensure they do not lead to toxicity issues.” But things seem promising on that front.
“Generally, iron oxide magnetic nanoparticles are considered biocompatible and have been already employed in various environmental and biomedical applications,” said Xia. “In addition, in our case, the nanoparticles can be easily collected by a permanent magnet at the end of the process, ensuring that no particles are left to contaminate the water.”
Not all plastics polluting our waters are positively charged, though. Many are negatively charged under normal aquatic conditions, meaning MARs wouldn’t be able to capture them through their current built-in electrostatic interactions.
“Our system on the initial experimental stage is kind of limited because MARs only could capture positively charged plastics,” said Xia. “In the future, I also would like to develop a system that can capture negatively charged micro/nanoplastics. But now, I need time to think about it.
“I think the utilization of natural sources, like algae cells, to accomplish specific tasks is highly promising. I believe if developed enough, MARs would be sufficient to deal with the recovery of micro/nanoplastics.
“It’s possible they could complement other methods rather than entirely replace them. This may include their combination with other functional nanoparticles, which can allow MARs to perform other tasks.”
4155/v





















